HeartLung’s Leadership Featured on Cardiac Wire Show to Discuss New AI for Early Cardiac Chamber Enlargement Detection
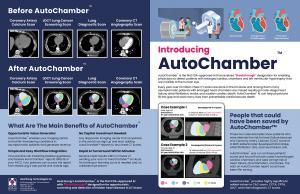
FDA Breakthrough-designated AutoChamber™ AI uses routine CT scans to detect early cardiac chamber enlargement and prevent serious heart disease.
HOUSTON, TX, UNITED STATES, August 12, 2025 /EINPresswire.com/ -- In a compelling new interview featured on The Cardiac Wire Show, HeartLung Technologies’ founder Dr. Morteza Naghavi joined a distinguished panel of cardiovascular, radiology, and preventive medicine experts to discuss AutoChamber™—HeartLung’s FDA-cleared AI platform for measuring cardiac chamber sizes and detecting asymptomatic enlargement linked to heart failure, atrial fibrillation, and stroke.Joining Dr. Naghavi were world-renowned leaders in cardiovascular research and imaging:
• Dr. Robert Kloner, cardiologist, professor of medicine at the Keck School of Medicine at USC, and Director of Cardiovascular Research at the Huntington Medical Research Institute.
• Dr. Nathan Wong, professor and director of the Heart Disease Prevention Program at UC Irvine, internationally recognized for his work in preventive cardiology and cardiovascular epidemiology.
• Dr. Claudia Henschke and Dr. David Yankelevitz, pioneers in CT lung cancer screening at Mount Sinai and co-founders of the International Early Lung Cancer Action Program (I-ELCAP).
The discussion highlighted how AutoChamber™ leverages routine non-contrast CT scans—including those performed for lung cancer screening, calcium scoring, and other clinical reasons—to automatically measure heart chamber volumes and detect enlargement years before symptoms develop.
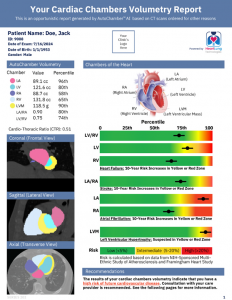
“Enlarged left atrium, left ventricle, or increased LV mass are strong predictors of future cardiovascular events, but historically they’ve gone undetected on non-contrast CT,” said Dr. Kloner. “AutoChamber™ brings a new dimension to preventive imaging—turning existing scans into a powerful tool for early detection and intervention.”
Dr. Wong emphasized the tool’s value in both diabetic and non-diabetic populations: “People with diabetes and enlarged chambers are at very high risk for heart failure, but what’s striking is that non-diabetics with chamber enlargement are at similar risk. AutoChamber™ helps us identify high-risk patients across the board and intervene earlier.”
Drs. Henschke and Yankelevitz underscored the broader public health opportunity: “Low-dose CT lung cancer screening already saves lives by detecting cancer early. By adding AutoChamber™, we can also identify silent heart disease in the same exam—preventing more deaths from cardiovascular disease than from lung cancer itself.”
From a technical standpoint, Dr. Naghavi noted that AutoChamber™ can process a full CT scan and deliver a volumetric chamber analysis in under a minute—something that would take a human reader days to perform manually. Medicare has recently approved reimbursement for AutoChamber™, further supporting adoption in both hospital and outpatient settings.
The panel agreed that, as with any breakthrough technology, the biggest hurdles are education and integration. “Providers need to know this exists and understand its value,” said Dr. Naghavi. “We now have an FDA-cleared, reimbursable way to detect heart disease years before symptoms—there’s no reason not to use it.”
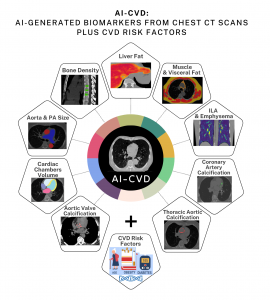
The Cardiac Wire Show interview underscores HeartLung’s vision for comprehensive, AI-powered preventive imaging, where multiple disease indicators—including cardiac chamber enlargement, coronary calcium, osteoporosis, and more—are identified by HeartLung’s AI-CVD™ technology from a single low-dose CT scan.
AutoChamber has received FDA Breakthrough Device designation and is launching clinically alongside HeartLung’s other tools, including AutoBMD for bone density screening.
About AutoChamber™ AI:
HeartLung’s AutoChamber™ is designed to work with both non-contrast and contrast-enhanced chest CT scans, providing estimates of cardiac volume, cardiac chambers volumes, and left ventricular wall mass. This AI-powered tool detects cardiomegaly and enlarged individual cardiac chambers, including the left atrium (LA) and left ventricle (LV), which are often missed in routine scans. By identifying these conditions early, AutoChamber™ AI helps prevent life-threatening diseases like stroke, heart failure, and atrial fibrillation. It has received FDA "Breakthrough Designation" for its ability to identify enlarged cardiac chambers and left ventricular hypertrophy in non-contrast chest CT scans.
About AI-CVD™
Comprehensive AI Solution for Cardiovascular Disease Prevention
HeartLung Technologies' AutoChamber™ and AutoBMD™ are integral components of AI-CVD™, a suite of AI-powered tools designed to detect and prevent cardiovascular disease. AI-CVD™ leverages advanced algorithms to analyze CT scans, identifying hidden heart risks and enabling early intervention. This comprehensive approach underscores HeartLung's commitment to revolutionizing preventive healthcare through innovative AI technologies.
About HeartLung Technologies
HeartLung leverages AI technology for the early detection and prevention of heart disease, lung cancer, emphysema/COPD, osteoporosis, myosteatosis, fatty liver disease, and other life-threatening conditions. HeartLung has received FDA "Breakthrough Designation" for AutoChamber™, an AI tool that identifies enlarged cardiac chambers and left ventricular hypertrophy in non-contrast chest CT scans, which are typically undetectable by the human eye. The AutoChamber™ AI also works on low-dose CT for lung cancer screening as well as contrast-enhanced coronary CT angiography (CCTA) scans. Additionally, HeartLung has obtained FDA 510(k) clearance for AutoBMD™, the only DEXA-equivalent, CT-based opportunistic osteoporosis screening approved by the FDA, applicable to over 25 million CT scans annually and reimbursed by Medicare. HeartLung is also awaiting FDA approval for AI-CVD™, a suite of AI modules including AI-CAC™ (AI-enabled Coronary Artery Calcium Scoring), aimed at early detection and prevention of cardiovascular disease using widely available CT scans.
Marlon Montes
HeartLung Technologies
+1 310-510-6004
email us here
Visit us on social media:
LinkedIn
X
The Cardiac Wire Show - A Deep Dive into HeartLung Technologies' AutoChamber AI
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.